Definition
Ciguatera is a widespread food-borne disease.
It is a form of ichtyosarcotoxism whereby humans are poisoned by eating fish intoxicated with toxins generated by micro-algae at the base of the food chain.
The main algae involved in the production of toxins is the dinoflagellate Gambierdiscus toxicus (Adachi and Fukyo, 1979)
although other species are also presumed to be associated with the disease but their role is more circumstantial. (Gramade and Dickey, 1990; Bomber, 1991)
History
As stated in Bagnis (1981), the history of the disease dates back as far as 1555, when the first case was reported in the West Indies. The term itself originates from the marine snail "cigua", due to the fact that the symptoms of the disease are similar to those resulting from snail-consumption. It was first reported in the Indian Ocean in 1601. In the South Pacific Ocean, the first noted case of ciguatera was in 1770 when the crew of the Fernandez de Quiros became intoxicated. In 1776, Cook and Forster had noted in their ships journals further cases of poisoning by fish coming from the Pacific. In French Polynesia, the historic references of intoxicated fish date back to 1792.
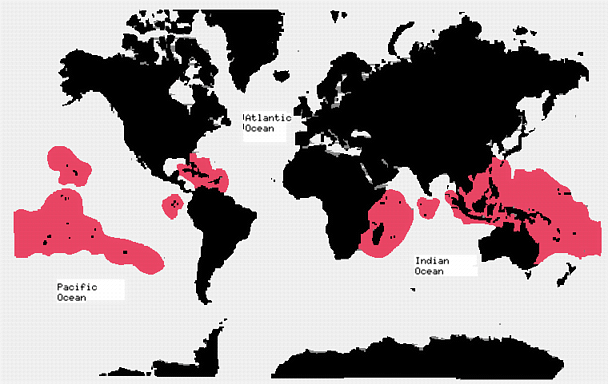
Distribution
Ciguatera is widespread around the circumtropics and globally around 20,000-50,000 people annually are estimated to suffer from the poisoning (Lewis, 1992; Yasumoto and Satake, 1996). The geographical distribution of ciguatera is mainly confined to a circumglobal belt of latitude 35°N-35°S. The main areas are in the Carribean, the Indian and the Pacific Oceans. However, even within this range there is a large variability in its occurance. For example, it is almost unknown in the Maldives, the Seychelles, Guam, the Solomans, Wallis, Futuna and several other places. On the other hand there are areas that are toxic, including the area north of Reunion Island and Mauritius.There are now also cases of ciguatera in the USA and Canada as a result of tourism to contaminated areas and from the export of intoxicated fish.
Symptoms
Symptoms are rarely fatal, although mortality can result. This is normally brought about by the consumption of the most toxic part of the fish, like the liver or the roe. The severity of the symptoms varies widely, as do the symptoms themselves. Normally, the victim will suffer from parasthesia, head-ache and vertigo in the first two to twelve hours after ingestion. Following this, symptoms include nausea, vomitting, a fall in blood pressure, irregular heart beat and an increase in parasympathetic activity. In severe cases paralysis, comatosis and death can result.Natural antidotes against ciguatera have been proposed; plant remedies have been traditionally used. Laurent et al. (1993) reported a list of about 100 plants known in New Caledonia and Vanuatu, but tests have yet to be done to determine their effective detoxification action. Currently, mannitol is the advised treatment in the acute phase of the intoxivation (Lewis, 1992). An intravenous injection at a dose of 0.5 to 1.0g/kg body weight has recently been used with success ( Palafox et al., 1992).
Cause
It was not until 1958 that Randall first specutaled that the primary vector of ciguatera was a benthic micro-organism and it wasn't until two decades later in 1977 that Bagnis et al. and then Yasumoto et al. (1979) suggested that this organism was a dinoflagellate. It is now known that the cause of ciguatera lies with the production of toxins by benthic dinoflagellates, in particular, the species Gambierdiscus toxicus. These dinoflagellates become problematic when populations increase after the destruction of coral reefs, as this provides an ideal habitat for population expansion. When a reef is destroyed, either by natural factors, such as storms, earthquakes (Bagnis and Denizot, 1978; Bagnis, 1994) or a rise in temperature (bleaching), or by anthropogenic influence such as dredging work, construction of ports and peers (Bagnis, 1968, 1987; Koike et al., 1991) and eutrophication, the freshly denuded surfaces are colonised by opportunistic macro-algae such as Halymenia sp., Portieria sp., Turbinaria sp. and Sargassum sp. etc. . These macro-algae are the hosts for the dinoflagellates responsible for ciguatera as they provide support and some of them excrete beneficial substances such as polyphenols, nitrogenous compounds and vitamins (Bomber et al., 1989) or inhibitive substances towards the different epiphytic species. It seems that the different macro-algal species are associated with different dinoflagellate species. For example, G.toxicus growth has been found to be stimulated by Turbunaria ornata.(Grzebyk et al., 1994) while P.lima, P.concavum and O.lenticularlis are associated with filamentous green algae (Kohler and Kohler, 1992). Generally, there is a preference for red algae. Turf algae, like Jania and Amphiroa are also preferential substrates for ciguateric dinoflagellates. Gambierdiscus toxicus has been observed on drift algae (Bomber et al., 1988) Suitable light conditions and salinity as well as the availability of a growth stimulant are also important for the spatial distribution of these protists.These dinofllagellates are not just epiphytic, some are benthic-detrital, like P.mexicamum, P.rueztlerianum, P.foraminosum, P.maculosum, P.hoffmannianum, P.emarginatum, Coolia monotis and Amphidinium sp., others are sand-dwellling like P.lima, P.sculptile, P.areanarium, and P.hoffmannianum while others are bloom-forming such as P.elegans and also P.mexicamum (Faust, 1995).
Herbivorous fish, such as the surgeon fish (Ctenochaetus striatus) and the parrot fish (Scaridae) consume the dinoflagellates and their toxins and so become toxic themselves. As larger, carnivorous fish ingest these fish, the toxins become concentrated and biotransformed. These carnivorous fish include species such as Moray eels (Murenidae), Jacks (Carangidae), Sea-bass (Serranidae) and Barracuda (Sphyraenidae). More than 425 species, from 60 different families have been found to carry ciguatera toxins (Bruslé 1997). Invertebrates and shellfish can also become toxic and transfer toxins up the food chain. For example; cladocera species and shrimps have been implicated in the poisoning of fish as have gastropod species and molluscs. (Russel and Egen, 1991) This transfer of toxins up through the trophic levels is known as the "food chain theory". (Glaziou and Legrand, 1994)
Dinoflagellates and toxins
The main dinoflagellate thought to be the causitive agent of ciguatera is Gambierdiscus toxicus (Adachi and Fukyo, 1979; Fukuyo and Ishimaru, 1986). There are also other species involved, including Prorocentrum lima, P. concavum, P. hoffmannianum, P. cassubicum, P. mexicanum, P.miniumum, Ostreopsis lenticularis, O. siamensis, O. ovata, O. mascarensis, O.heptagona, Amphidinium carteri, A. klebsii and Coolia montis. These different species produce a number of toxins and this is thought to be the cause of the multiplicity of symptoms in the pathology of ciguatera (Bomber, 1991).Ciguateric fish poisoning involves multiple toxins (Gramade and Dickey, 1990). The principal toxins involved are natual lipidic toxins known as ciguatoxins (CTX). However, maitotoxin (MTX), scaritoxin (STX) and okadaic acid are also involved. Maitotoxin, a water soluble compound is associated with the digestive tract of the herbivorous fish whilst ciguatoxins are associated with the viscera but also with the liver and muscle of the herbivorous and carnivorous fish.
G. toxicus can produce two types of toxin but other types appear as they are transferred up the food-chain and undergo acidic digestion and biotransformation. G. toxicus also produces maitotoxin which is the most potent marine toxin and gambiertoxin (GTX) which is a precursor for ciguatoxin. This is a less polar type of ciguatoxin, which can be oxidatively metabolised in fish to produce ciguatoxin.
P. lima , P. concavum and P. hoffmannianum are known to produce okadaic acid and are thought to be the cause of the gastro-intestinal symptoms. (Bomber, 1991)
| Species | Compound | Action of toxin |
| G.toxicus | maitotoxin ciguatoxin |
neurotoxin |
| C.monotis | unnamed | hemolytic |
| O.lenticularis | ostreotoxin | neurotoxin |
| O.heptagona | unknown | - |
| O.ovata | unknown | hemolytic |
| O.siamensis | unknown | hemolytic |
| O.mascarenensis | maitotoxin | neurotoxin |
| P.concavum | okadaic acid | diarrhetic |
| P.lima | okadaic acid | diarrhetic |
| P.mexicanum | fast acting toxin | hemolytic |
| P.hoffmanium | okadaic acid | diarrhetic |
The presence of these dinoflagellates does not necessarily mean ciguatera will be present. For example, in the Lagoon of Mayotte numbers of G toxicus have been increasing since 1988, but as yet, no ciguatera poisoning has been found (Grzebyk, 1993). However, it has been said that the initial requirement for an iniation of ciguatera in a region is an increase in the population of G. toxicus (Gillespie et al., 1985). Despite this, even when numbers increase the population can still be non-toxic. It has been hypothesised that CTX is not always a growth product of G toxicus but that a specific stimulus may be required to begin the sequence of events leading to the formation of CTX (Gillespie et al., 1985; Faust, 1995). There is also the hypothesis that genetic differences in populations of dinoflagellates result in lack of production of ciguatoxin precursors (Lewis et al. 1988). It has also been suggested that these polyether toxins may be produced to serve as allelochemical agents directed against close competitors or pathogens or to inhibit grazers (Morton et al., 1994).
As well as the poor heath as a result of poisoning, ciguatera has profound social and economic impacts. The loss of working days due to illness negatively effects the economy. To add to this, the prohibition of fish sales causes massive losses for the fish industry. There is also a large negative impact on aquaculture and tourism as a result of ciguatera.
References
- Adachi R. and Fukuyo Y., 1979. The thecal structure of a marine toxic dinoflagellate Gambierdiscis toxicus gen. and sp. nov. collected in a ciguatera endemic area. Bull. Jap. Soc. Sci. Fish. 45, 67-71.
- Bagnis R., 1969. Naissance et développement d'une flambée de ciguatera dans un atoll des Tuamotu. Rev. Corps Santé Armées, 10: 783-795.
- Bagnis R., 1981. L'ichtyosarcotoxisme de type ciguatera: processus biologiques connus et perspectives ay seuil des années 80. Ann. Inst. Océanogr. Paris, 57: 5-24.
- Bagnis R., 1987. Ciguatera fish poisoning: an objective witness of the coral reef stress. In: Human impacts on coral reefs: facts and recommendations. Salvat B.(Ed.). pp. 241-253.
- Bagnis R., 1994. Natural versus anthropogenic disturbances to coral reef. Comparison in epideiological patterns of ciguatera. Mem. Queensl. Mus. 34: 455-460.
- Bagnis R. and Denizot M., 1978. La ciguatera aux Iles Marquises: aspects humains et biomarins. Cah. Pacif. 21: 293-314.
- Bagnis R., Chanteau S. and Yasumoto T., 1977. Un agent étiologique vraisemblable de la ciguatera. CR Acad. Sci. 285: 105-108. species in coastal waters of south west Peurto Rico Proceedings of the 5th International Coral reef congress, Tahiti. vol. 4, 412-417.
- Bomber J.W., 1991. Toxigenisis in Dinoflagellates - genetics and physical factors. In : Ciguatera Seafood toxins. D.Millar (Ed.) CRC Press, pp 135-170.
- Bomber J. W., Rubio M. G. and Norris D. R., 1989. Epiphytism of dinoflagellates associated with the disease ciguatera: substrate specifity and nutrition. Phycologia, 28: 360-368.
- Bomber J. W., Morton S. L., Babinchak J. A., Norris D. R. and Norton J. G., 1988. Epiphtic dinoflagellates of drift algae-another toxigenic community in the ciguatera food chain. Bull. Mar. Sci. 43: 204-214.
- Bruslé J., 1997. Ciguatera fish poisoning - a review. Sanitary and ecomonic aspects. INSERM, Paris. 147p.
- Faust M.A., 1995. Benthic, toxic Dinoflagellates: an overview. In: Harmful Marine Algal Blooms. Lassus P., Arzul G., Erard-Le Denn E., Gentien P. and Marcaillon - Le Baut C. (Eds.) Lavoisier, Paris, pp.847-854.
- Fukuyo Y. and Ishimaru T., 1986. in Maclean, J.L., Dizon, L.B. and Hosillis, L.V., (Eds.). The First Asian fisheries Forum, Asian Fisheries Society, Manila, Philippines. P.307-310.
- Gillespie N., Lewis R., Burke J. and Holmes M., 1985. The significance of the absence of ciguatera in a wild population of Gambierdiscus toxicus. Proceedings of the 5th International Coral reef congress, Tahiti, 4, 437-441.
- Glaziou P. and Legrand A. M., 1994. The epidemiology of ciguatera fish poisoning. Toxicon, 31, 1151-1154.
- Gramade H.R. and Dickey R. 1990. Recent advances in ciguatera fish poisoning research. 15. Annu. Conf. : Tropical and Subtropical Fisheries Technological Conf. of the Americas, in 2. Joint Meet. with Atlantic Fisheries Technology Conf. , Orlando, FL. USA
- Grzebyk D., 1993. Dinoflagellés marins toxiques: biologie, écophysiologie, These, Univerisite D'Aix-Marseille II. p202
- Grzebyk D., Berland B., Thomassin B.A., Bosi C. and Arnoux A. 1994. The ecology of ciguateic dinoflagellates in the coral reef complex of Mayotte Island (S.W Indian ocean). J. Exp. Mar. Biol. Ecol,
- Kohler S.T. and Kohler C.C. 1992. Dead bleached coral provides new surfaces for dinoflagellates implicated in ciguatera fish poisoning. Env. Biol. Fish., 34, 4, 413-416.
- Koike K., Isimaru T. and Murana M. 1991. Distributions of benthic dinoflagellates in Akajima island, Okinawa, Japan. Nippon Suisan Gakkaishi, 57: 2261-2264.
- Laurent D., Bourdy G., Amade P., Cabalion P. and Bourret D. 1993. La gratte ou ciguatera. Ses remedes traditionnels dans le Pacifique Sud. Ed ORSTOM, Paris, 150p.
- Lewis R. J. 1992a. Socio-economic impact and management of ciguatera in the Pacific. 4th Int. Conf. on ciguatera fish poisoning, Tahiti. p.11
- Lewis R. J. 1992b Mannitol : the treatment of choice in the acute phase of ciguatera; SPC Ciguatera Inf. Bull., 2, 9-10.
- Lewis R. J., Chaloupka M. Y., Gillespie N. C. and Holmes M. J. 1988. An analysis of the human response to ciguatera in Australia. Proceed. 6th Int. Coral Reef Symp., 3, 67-72.
- Morton S. L. Bomber J. W. Tindall P. M. 1994. Environmental effects on the production of Okadaic acid from Prorocentrum hoffmannianum Faust I, temperature, light and salinity. J. Exp. Mar. Biol. Ecol., 178, 66-77.
- Palafox N., Jalm L. G., Pinano A. Z., Gulick T. M., Williams R. K. and Shatz L. J., 1992. Successful treatment of ciguatera fish poisoning with intravenous mannitol. 4th Int. Conf. on ciguatera fish poisoning, Tahiti. p.8
- Randell J. E. 1958. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. Gulf and Caribbean., 8: 236-267
- Russel F. E, and Egen N.B., 1991. Ciguatera fishes, ciguatoxin (CTX) and ciguatera poisoning. J. Toxicol. - toxin review. 10, 1, 37-62.
- Yasumoto T., Nakajima I., Oshima Y. and Bagnis R., 1979. Anew toxic dinoflagellate found in association with ciguatera. In: Toxic Dinoflagellate Blooms, Taylor and Seliger (Eds.) Elsevier NY, pp. 65-70.
- Yasumoto T. and Satake M. 1996. Chemistry, etiology and determination of methods of ciguatera toxins. J. Toxicol. Toxin Review. 15, 2, 91-107.
Attention... page non mise à jour depuis le 19 Jan 2006

